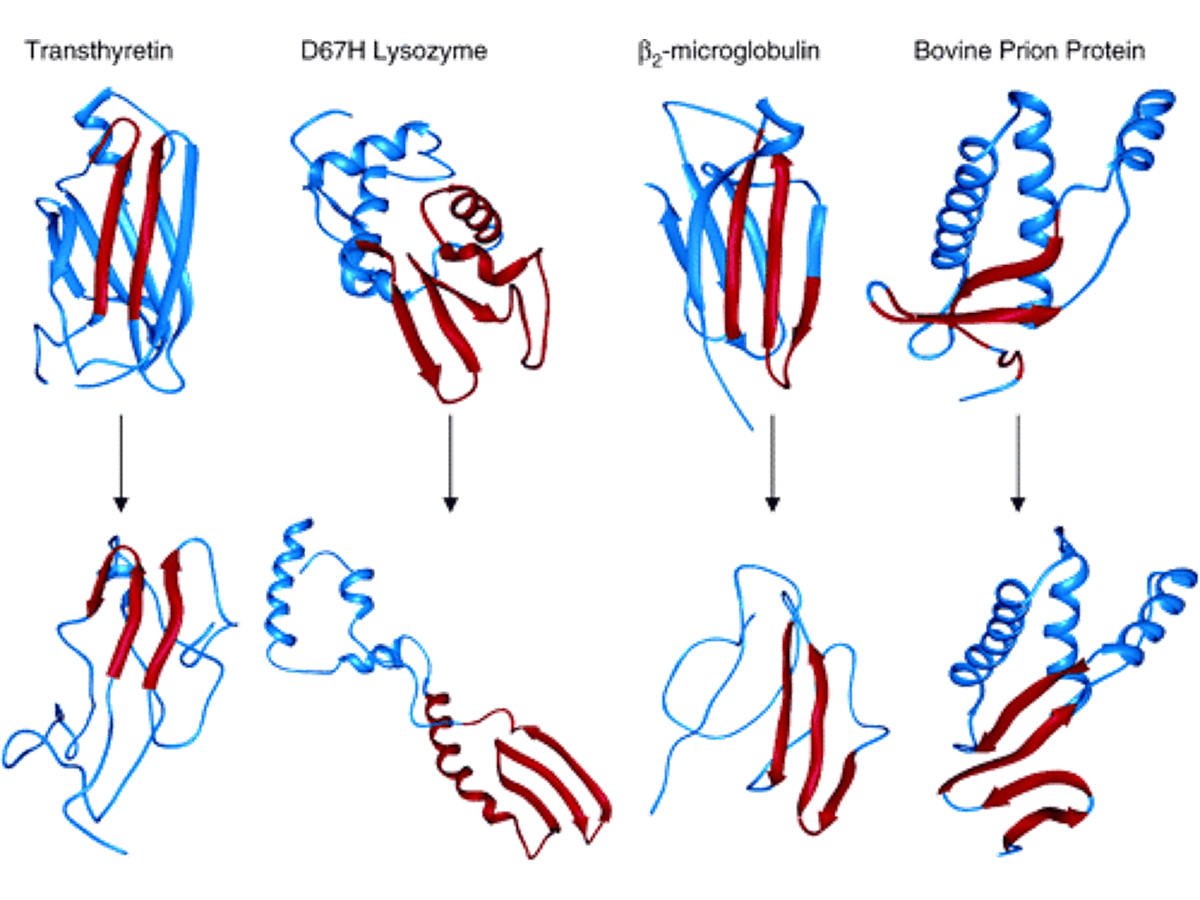
Native crystal structures for four amyloidogenic proteins (top) and their conversion to α-sheet (red strands, lower structures) during MD simulations at low pH with all native disulfide bonds intact. (image source)
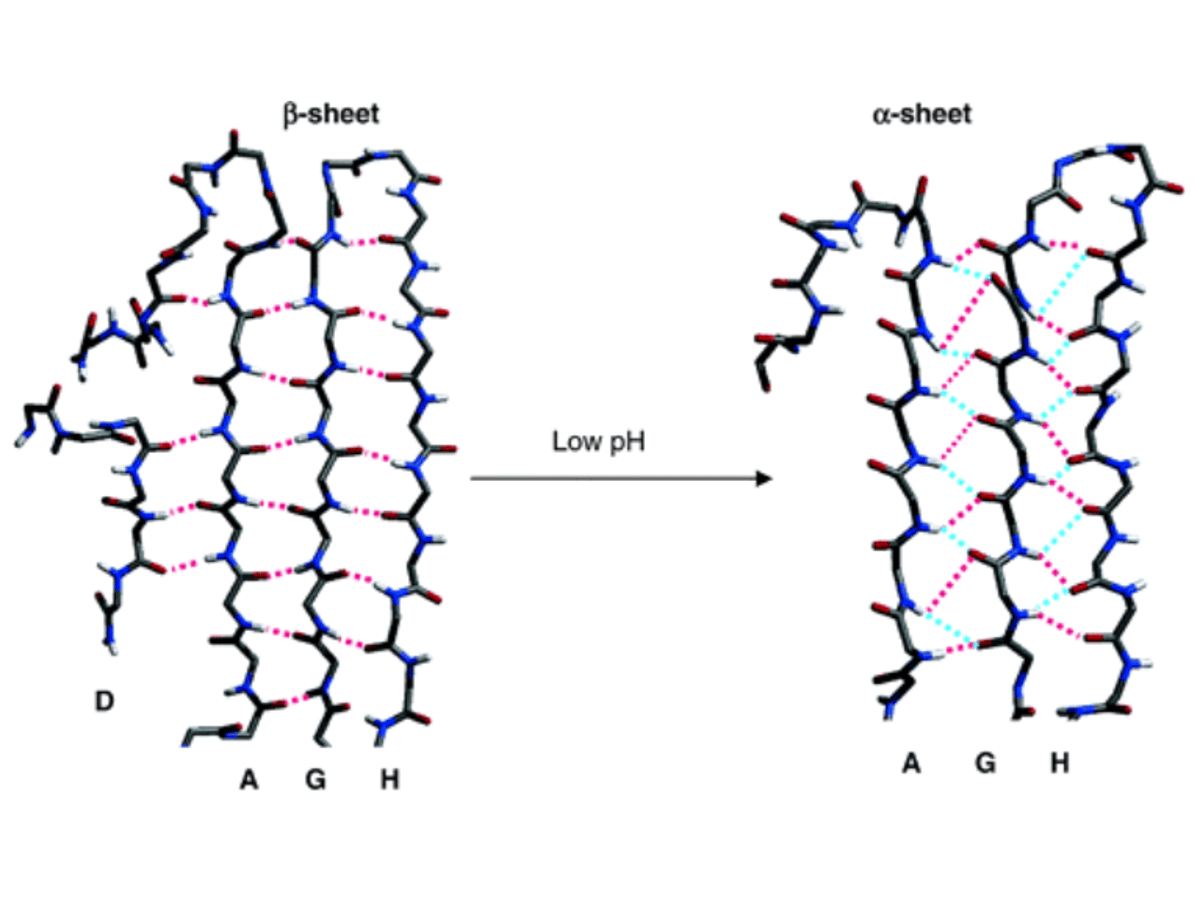
The DAGH β-sheet from the transthyretin crystal structure and after MD at low pH. (image source)
α-Strand and α-sheet structure in lysozyme: (A) α-strand structure in hen egg white lysozyme crystal structure; (B) conversion to α-sheet in MD simulation of human variant at low pH and a model of an α-sheet aggregate. (image source)
There are now over 50 known amyloid diseases that affect a variety of tissues throughout the body. Amyloid diseases are characterized by the aggregation of peptide/protein monomers into toxic, soluble oligomers, and eventually mature, insoluble plaques that are relatively nontoxic. We have proposed that α-sheet plays a critical role in both the aggregation and toxicity of soluble oligomers.
Our work with disease systems revolves around the design of templated α-sheet peptides that inhibit aggregation and mitigate toxicity of the amyloid species in vitro and in vivo. We use a variety of standard and novel techniques to help us define the role of α-sheet in amyloid diseases, including: spectroscopic characterizations; cell-based assays; testing in relevant animal disease models; and structure-based assays. We have found a strong correlation between α-sheet content and toxicity and are developing a plate-based Soluble Oligomer Binding Assay (SOBA) for detection of toxic species.