AFM image of synthetic PSMα1 amyloid fibrils. (image source)
Healthcare-associated infections (HAI) are the most common adverse event in healthcare delivery worldwide and a significant contributor to mortality and financial losses. Because microbial infections typically occur on surfaces – e.g., prosthetic heart valves, urinary and intravascular catheters, orthopedic implants – more than half of HAIs are associated with biofilm formation. Biofilms are surface-associated, spatially structured bacterial communities, and the high density of these environments enables specialized intercellular communication via quorum sensing. Microbes in biofilms also produce an extracellular matrix (EM) to mediate attachment, immobilize the cells, and promote metabolite transport. The protective coating of the EM shields bacteria from exogenous compounds, reducing their susceptibility to antibiotics by several orders of magnitude compared to free-swimming microbes. Antibiotic resistant bacteria are now implicated in at least 14% of all HAI [CDC, 2016].
The EM is comprised of hydrated polysaccharides, extracellular DNA, and proteins. Proteins in the EM take on a variety of roles, but a growing body of research seeks to understand the role of amyloid fibrils in this complex biological material. Extracellular deposition of amyloid has long been associated with protein misfolding and neurodegenerative conditions such as Alzheimer’s disease, but recent research suggests that many bacteria produce extracellular amyloid fibrils to reinforce the biofilm and resist dispersion by chemical or mechanical agents. These fibrils are biochemically similar to those formed in mammalian disease, but unlike pathological amyloids, bacterial amyloids are intentionally produced to play a functional role. The conservation of the amyloid fold through millions of years of evolution likely relies on amyloids’ universal characteristics – such as backbone hydrogen bonding – rather than specific side chain chemistries. Additionally, amyloid fibrils polymerize in the absence of an energy source, so they serve as a metabolically advantageous molecular scaffold despite the limited resources of the EM environment.
The FapC protein from P. aeruginosa aggregates to form amyloid fibrils. (image source)
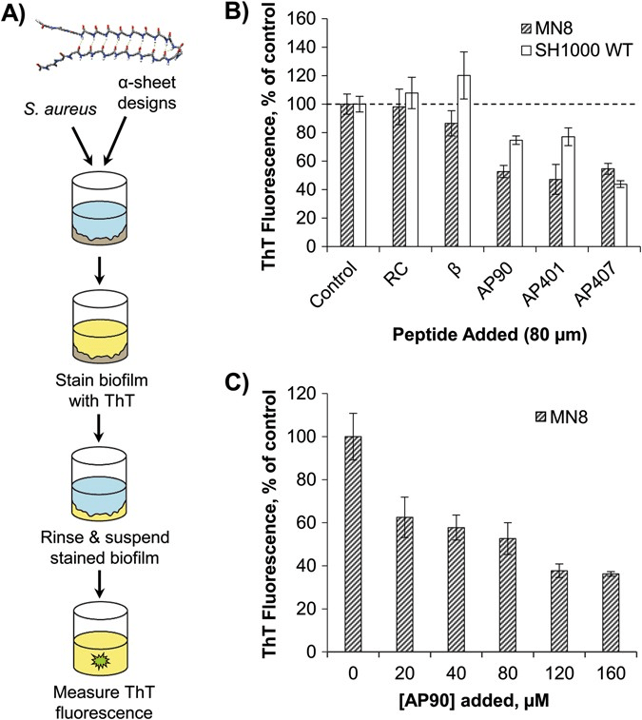
Screening of designed peptides for inhibition of amyloid formation in S. aureus biofilms. (image source)
The ability of functional amyloid fibrils to influence biofilm development has made them an attractive target for therapeutic intervention, but efforts to suppress amyloid formation in biofilms remain quite limited. Thus, we developed and tested several peptide designs for their ability to inhibit bacterial amyloid formation in three pathological biofilms – S. aureus, P. aeruginosa, and E. coli. Our design approach, based on unique “α-sheet” structural motifs observed in molecular dynamics (MD) simulations, targets pre-amyloid oligomers formed on the pathway to fibril formation. These results support our hypothesis that α-sheet is a generic structure formed during amyloidogenesis independent of source, sequence and structure. The inhibitory effect of designed α-sheet peptides is shown to persist even in the complex matrix of mature biofilms.