Research
Crystal nucleation, growth, and dissolution
Crystallization is a critically important phenomenon, with relevance to materials engineering (materials synthesis), geochemistry (mineralization), and biology (protein assembly and biomineralization). We combine modern in-situ microscopies (e.g. in-situ AFM, in-situ TEM, and cryogenic TEM), and with advanced modeling tools (e.g. molecular dynamics and Monte Carlo simulations) to investigate the fundamental mechanisms of crystallization, across a wide diversity of systems. This suite of techniques allows us to investigate crystallization from the earliest stages (e.g. the formation of transient molecular clusters that serve as crystal nuclei) to the ultimate formation of complex hierarchical crystalline structures. A particular focus involves the emergence of complex growth pathways that involve cluster assembly, metastable intermediates, and particle aggregation.
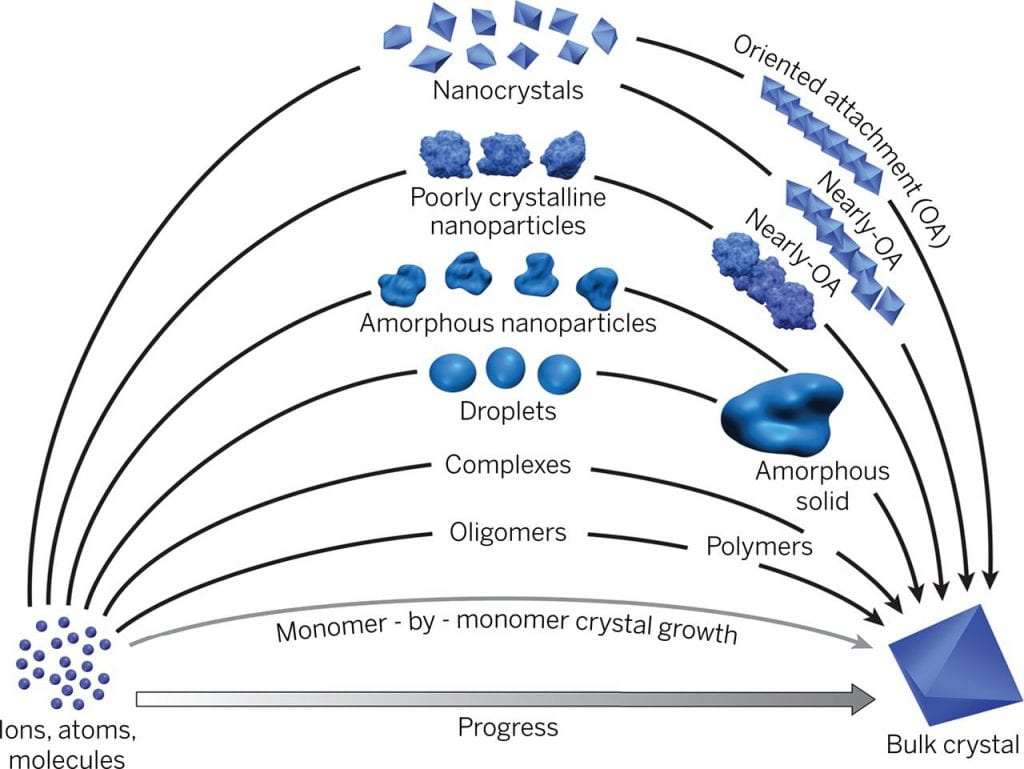
Nanoparticle assembly and oriented attachment
Numerous pieces of evidence are challenging the traditional interpretations of crystallization. Crystallization involving particle attachment has been demonstrated in my different materials with fascinating structure and order. However, many basic aspects regarding their formation process and driving force are largely unknown. Our goal is to combine multiple imaging and spectroscopic techniques to answer questions like, How does phase transformation happen during crystallization? What drives the order development during oriented attachment and how? What’s the fundamental parameter governing the crystallization pathway?
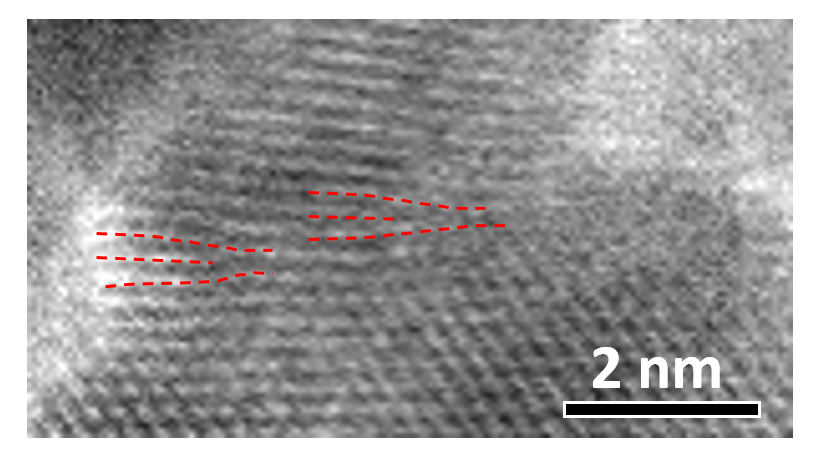
Macromolecular self-assembly
Self-assembly is an efficient bottom-up method to organize matters across scales. Researchers have utilized self-assembly to design and synthesize a wide range of biomaterials and hybrid materials with diverse structures and functions. In our group, we use in situ atomic force microscopy to observe and monitor the assembly processes and reveal their assembly pathways and mechanisms. These kinetic and physical understandings will lead to novel strategies for designing molecular assembled systems and enable us to precisely control the morphologies and phases of these assembled materials.

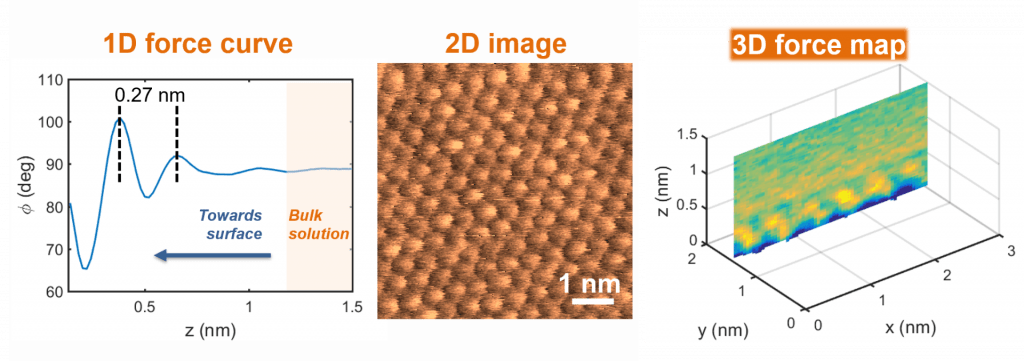
Structure of solid-liquid interfaces
Many interesting phenomena occur at interfaces, from catalysis and electron transfer to ion adsorption and particle attachment. However, the molecular details of solid-liquid interfaces remain poorly understood. We are investigating interfacial solution structure and learning how the arrangement of ions and solvent molecules at a crystal surface is influenced by crystallography, solution conditions, and surface chemistry. For this purpose, we use a recently developed atomic force microscopy technique, known as 3D force mapping, coupled to molecular dynamics simulations, which enables us to visualize solution structure with sub-nanometer resolution.
Biomineralization, biomimetic crystallization, and mineral-organic interactions
The control that living organisms exert on crystallization is extraordinary in terms of location, orientation, size, morphology, and phase, a stark contrast to the outcomes of synthetic crystallization. However, the role of many organic and inorganic components and mechanisms by which biomineralization occurs remains elusive, such as the hierarchical structure of apatite in tooth enamel or calcite in coccolithophores. We systematically investigate the role of the organic components by exposing them to physiologically relevant solutions. Our group probes this dynamic environment at the nanoscale using in situ AFM and TEM to unravel the stereochemical, thermodynamic, and kinetic parameters governing mineral nucleation and growth to build knowledge on naturally occurring processes.
