|
Andrea Wills
Associate Professor of Biochemistry
B.A. 2002, Pomona College
Ph.D. 2009, University of California, Berkeley
|
Honors
- 2014 John Gurdon Prize (for best trainee talk), 15th International Xenopus Meeting. Pacific Grove CA.
- 2014 Hilde Mangold Postdoctoral Prize (for best talk). 73rd Annual SDB meeting. Seattle, WA.
- 2010-2013 NIH NRSA fellowship
Research
Why is regeneration of adult human tissues so limited, when other vertebrates are readily able to regenerate complex tissues like the spinal cord, cartilage, and limb? Our goal is to define the genetic pathways and epigenetic mechanisms that govern differentiation of different cell types during regeneration and embryogenesis. We employ a combination of genomics, embryology and cell biology using the diploid frog Xenopus tropicalis as a model for regeneration. Tadpoles are able to rapidly regenerate tissues of the nervous system, musculature, cartilage and vasculature after injury, but lose this regeneration competence as metamorphosis progresses. We are interested in all aspects of regeneration, but two themes are of particular interest to the lab:
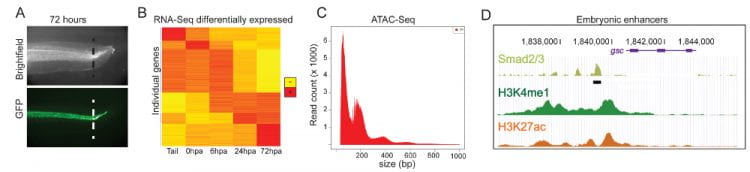
Do embryogenesis and regeneration use the same gene regulatory mechanisms? During embryonic development, cells acquire specific fates through progressive differentiation from pluripotent precursors, while during regeneration, new functional tissue must be established from tissues that have already differentiated. While many early developmental signaling pathways are re-activated in regeneration (Wnt, FGF, BMP, Notch, Shh and others), little is known about the gene regulatory environment that triggers their expression or restricts their tissue specificity. We use high-throughput sequencing approaches (RNA-Seq, ChIP-Seq, ATAC-Seq) to query these events over time in embryos, regenerating tissues, and isolated cell populations. We then take advantage of the large size and easy manipulability of X. tropicalis embryos to perturb the functions of specific genes and pathways. What limits regeneration competence? As the tadpole undergoes metamorphosis, its ability to regenerate declines. We are interested in defining the nature of this loss of competence. Is it cell-intrinsic, or a property of a changing signaling environment or inflammatory response in aging tissue? Can it be reversed? We are studying how gene expression and regulatory element usage change in response to injury in animals with varying regeneration competence.
Publications: