Membrane docking and fusion are the terminal events in vesicular transport. We have used the yeast vacuole as a model system to probe SNARE-mediated membrane fusion events in general, and fusion events in the endolysosomal system in particular.
During SNARE-mediated fusion, the two membranes are first brought into proximity. Membrane-anchored SNARE proteins then wind together in a concerted oligomerization and folding reaction — “zippering” — which leads to the formation of a trans-SNARE complex. Further zippering drives the membranes into contact, followed by merger of the two bilayers, fusion pore opening, and content mixing.
After fusion, the extremely stable cis-SNARE complex is disassembled through the action of an ATP-dependent chaperone system (Sec17 and Sec18), generating uncomplexed SNAREs that are re-energized for another round of fusion.
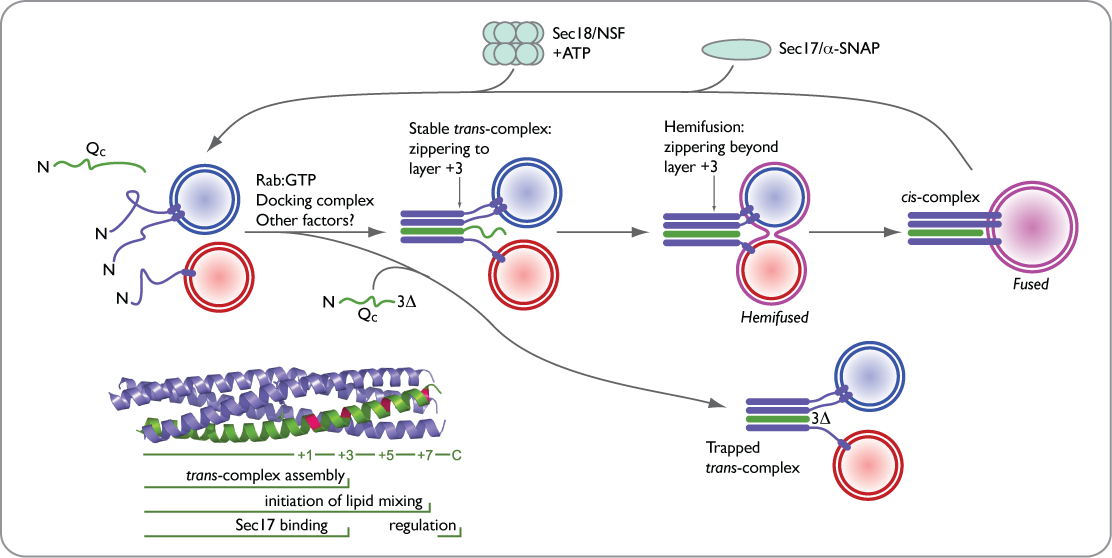
By preparing a series of truncations of a soluble SNARE, Vam7 (Qc), we have been able to slow and then stop an incipient fusion reaction, trapping the SNARE complex in its assembled and poised pre-fusion state. These powerful and extremely potent (apparent Km ~1 nM) reagents are being used to understand the composition and topology of the pre-fusion complex, and to deliver optical probes into the heart of the fusion machine.
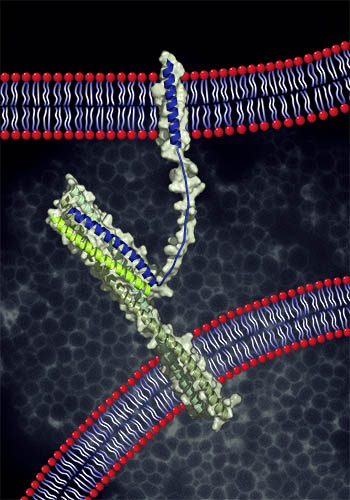
Of course, these cartoons are radical over-simplifications of docking and fusion as they occur in living cells. For each fusion reaction there are an array of other proteins: Rab small G proteins, Rab-binding tethering factors, assembly co-chaperones of the SM (Sec1-Munc18) family, and other factors including lipid and protein kinases.
Much of the last several years’ work in our lab (and many others) has been dedicated to understanding the functions of SNARE cofactors. We have focused most intently on the Vps-C tethering complexes, CORVET and HOPS. These complexes have overlapping subunit compositions, and operate at the late endosome and lysosome, respectively.
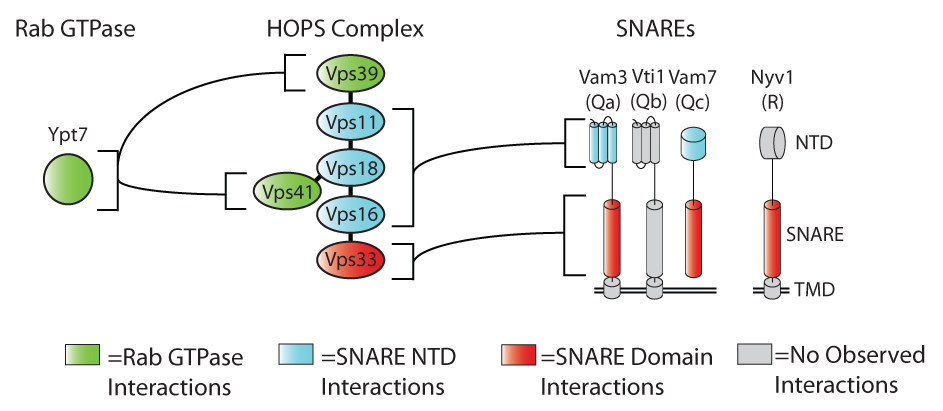
We have demonstrated that a pair of subunits unique to each complex serve as Rab-binding modules, while a common subunit, the SM protein Vps33, engages both individual SNARE domains and assembled (or partially assembled) SNARE complexes. In addition, there are accessory binding sites within the Vps-C complex which engage the N-terminal domains of at lest two of the SNAREs.
Going forward, we are addressing several questions:
• What is the mechanistic role of SM proteins in SNARE assembly and function?
• How do SM proteins and Sec17 (= α-SNAP) interact in cells? What are the functional consequences of these interactions?
• What is the relationship between SNARE zippering and fusion pore opening at sub-millisecond time scales?
• How are incipient fusion pores stabilized? What keeps them from collapsing?
• Does quality control occur in SNARE assembly? Are incorrect complexes inactivated, disassembled, or both? If so, what are the mechanisms through which this occurs?
• How is the process of vesicle formation coupled to vesicle docking and fusion at target membranes?
Key papers
Schwartz ML, Nickerson DP, Lobingier BT, Plemel RL, Duan M, Angers CG, Zick M, Merz AJ. 2017. Sec17 (α-SNAP) and an SM-tethering complex regulate the outcome of SNARE Zippering in vitro and in vivo. eLife 2017;6:e27396 doi: 10.7554/eLife.27396
Cattin-Ortolá J, Topalidou I, Dosey A, Merz AJ, Ailion M. 2017. The Dense-Core Vesicle Maturation Protein CCCP-1 Binds both RAB-2 and Membranes through a Conserved C-terminal Coiled-coil Domain Traffic, doi: 10.1111/tra.12507. Preprint version: bioArXiv
Song H, Orr AS, Duan M, Merz AJ, Wickner WT. 2017. Sec17/Sec18 act twice, enhancing fusion and then disassembling cis-SNARE complexes. eLife 2017;6:e26646 doi: 10.7554/eLife.26646
Zick M, Orr A, Schwartz ML, Merz AJ, Wickner WT. 2015. Sec17 can trigger fusion of trans-SNARE paired membranes without Sec18. Proceedings of the National Academy of Sciences, doi: 10.1073/pnas.1506409112.
Lobingier BT, Nickerson DP, Lo S-Y and Merz AJ. 2014. SM proteins Vps33 and Sly1 coassemble with Sec17 and SNARE complexes to oppose SNARE disassembly by Sec18. eLife, doi: 10.7554/eLife.02272 —pdf.
Lobingier BT and Merz AJ. 2012. The SM protein Vps33 binds to SNARE domains and the quaternary SNARE complex. Molecular Biology of the Cell 23:3429-37. —pdf
Lo S-Y, Brett CL, Plemel R, Vignali M, Fields S, Gonen T and Merz AJ. 2012. Intrinsic tethering activity of endosomal Rab proteins. Nature Structural & Molecular Biology, 19:40-47. *Faculty of 1000 Recommended. —pdf
Schwartz ML and Merz AJ. 2009. Capture and release of partially-zipped trans-SNARE complexes on intact organelles. Journal of Cell Biology 185:535-549. —pdf
Thorngren N, Collins KM, Fratti R, Wickner WT and Merz AJ. 2004. A soluble SNARE drives rapid docking, bypassing the need for ATP and Sec17/18p for vacuole fusion. EMBO Journal 23:2765-2776. —pdf
Merz AJ and Wickner WT. 2004. Resolution of organelle docking and fusion kinetics in a cell-free assay. Proceedings of the National Academy of Sciences 101:11549-11553. —pdf
Wang L, Seeley, S, Wickner WT and Merz AJ. 2002. Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell 108:357-69. —pdf