As cargos move through the endocytic pathway the organelles within which they reside undergo a maturation process. The lumen acidifies, phosphoinositide lipids on the organelle membrane are remodeled, and proteins that mark the early endosome are selectively removed while proteins characteristic of later ensodomes and lysosomes are added to the organelle through fusion events. At the same time a remarkable morphological transition is sequestering cargos into intraluminal vesicles (ILVs) to form the multivesicular body (MVB).
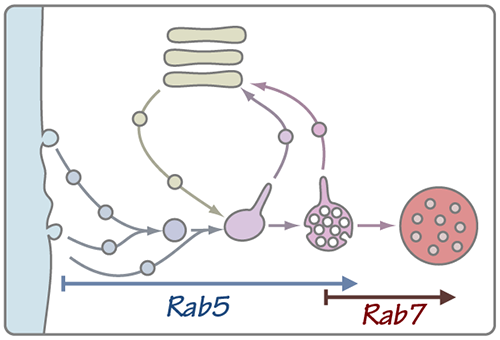
A key transition in this elaborate dance occurs when the late endosome ceases to accept inbound traffic from the plasma membrane and Golgi (processes dependent on small G proteins of the Rab5 family), and becomes competent to fuse with the terminal lysosomal compartment (an event controlled by Rab7). The transition from Rab5 to Rab7 signaling is clearly related to the formation of the MVB morphology, but the specific signals that trigger the Rab5-to-Rab7 transition are poorly understood.
Recently, we have identified key enzymes and other regulators that control the signaling state of Rab5 and Rab7. At the same time our collaborators in the Odorizzi group at the University of Colorado, Boulder have made substantial process in linking molecules that drive MVB formation to the Rab5-Rab7 transition. Going forward we will use genetic and cell biological approaches, electron microscopic (EM) tomography, and biochemical reconstitution to build and test models for this critical event in organelle maturation.
In parallel work we have studied the AP-3 transport pathway which carries cargo directly from the trans Golgi compartment to the terminal lysosomal vacuole, bypassing endosomes.
Lately we have been studying a remarkable mutation in an AP-3 subunit, which allows AP-3 to accumulate at the Golgi, but is defective at the vesicle budding step. The combination of EM tomography and 3D structured illumination microscopy (3D-SIM) has catalyzed a leap forward in our ability to analyze what is happening in both normal and AP-3 mutant cells. The micrograph below shows 3D-SIM of AP-3 vesicles (cyan) budding from the trans Golgi (red) in living wild type (normal) cells.
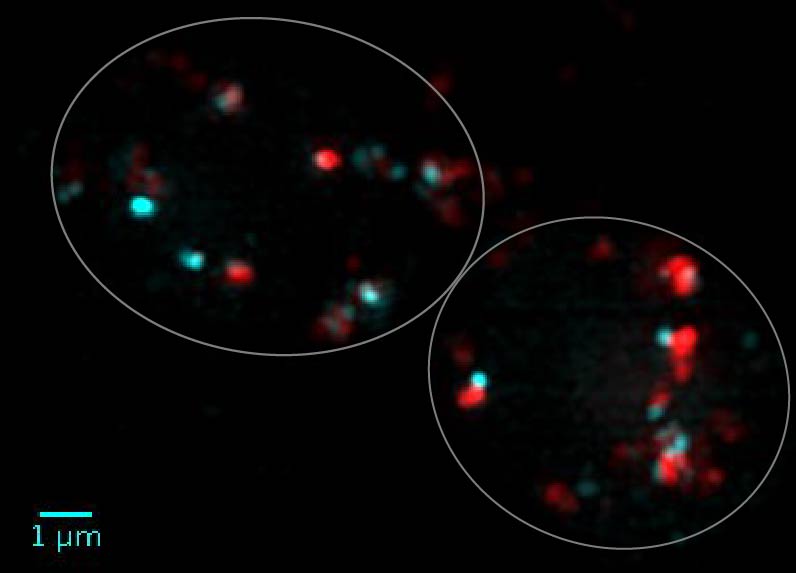
Another key result is that the AP-3 coat remains associated with the tranport vesicle until docking and fusion at the lysosomal target membrane (Angers and Merz, 2009; Schwartz et al., 2017). This is in contrast to conventional models where vesicle coats fall off shortly after a vesicle is formed and before docking and fusion.
Key papers
Schwartz ML, Nickerson DP, Lobingier BT, Plemel RL, Duan M, Angers CG, Zick M, Merz AJ. 2017. Sec17 (α-SNAP) and an SM-tethering complex regulate the outcome of SNARE Zippering in vitro and in vivo. eLife 2017;6:e27396 doi: 10.7554/eLife.27396
Shideler T, Nickerson DP, Merz AJ, Odorizzi G. 2015. Ubiquitin binding by the CUE domain promotes endosomal localization of the Rab5 GEF Vps9. Molecular Biology of the Cell — doi 10.1091/mbc.E14-06-1156.
Angers C and Merz AJ. 2011. Review: New links between vesicle coats and Rab-mediated vesicle targeting. Seminars in Cell & Developmental Biology 22:18-26.25. —pdf
Paulsel AL, Merz AJ, Nickerson DP. 2013. Vps9 family protein Muk1 is a second Rab5 GEF in budding yeast. Journal of Biological Chemistry 288:18162-71. —pdf.
Nickerson DP, *Russell MRG, *Lo SY Milnes J and Merz AJ. 2012. Termination of isoform-specific Vps21/Rab5 signaling at endolysosomal organelles by Msb3/Gyp3. Traffic 13:1411-28. *Equal contributors. —pdf
*Plemel RL, *Lobingier BT, Brett CL, Nickerson, DP, Angers CG, Paulsel A, Sprague D and Merz AJ. 2011. Subunit organization and Rab interactions of Vps-C complexes that control endolysosomal membrane traffic. Molecular Biology of the Cell 22:1353-63. *Equal contributors. —pdf
Angers C and Merz AJ. 2009. HOPS interacts with Apl5 at the vacuole membrane and is required for consumption of AP-3 transport vesicles. Molecular Biology of the Cell 20:4563-4574. –pdf
Brett CL, Plemel RL, Lobingier BT, Vignali M, Fields S and Merz AJ. 2008. Efficient termination of vacuolar Rab GTPase signaling requires coordinated action by a GAP and a protein kinase. Journal of Cell Biology 182:1141-51. *JCB featured paper; Faculty of 1000 Recommended. —pdf