trafficking probes: LUCID
The complexity of anterograde and retrograde traffic between the Golgi apparatus, the plasma membrane, autophagic vesicles, and the endolysosomal system is daunting. We are developing new probes and methods to quantitatively assess cargo flux through these pathways.
The first of these probes is the Luciferase reporter of intraluminal deposition, or LUCID. This probe facilitates the quantitative assay of integram membrane protein targeting to the lumen of the vacuole, via the late endosomal multivesicular body (MVB). Failure to package the luciferase-marked cargo into intraluminal vesicles of the MVB, or failure of the MVB to fuse with the hydrolytic vacuole, results in accumulation of luciferase in the cytoplasmic compartment.
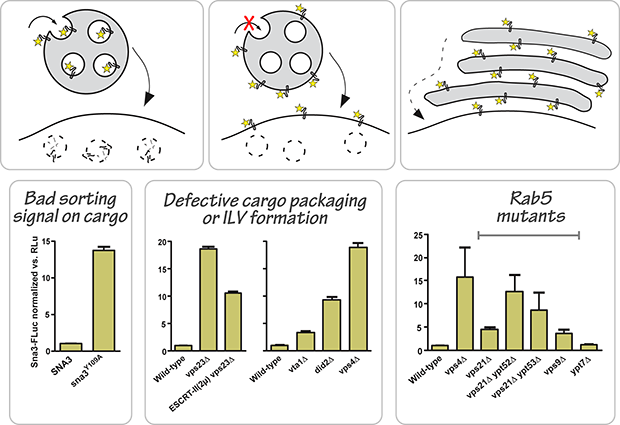
Our first description of the LUCID probe and assay was published in Nickerson, 2012, and additional cargo probes are under active development.
probes for protein catabolism
The nematode worm C. elegans is a premier system for dissecting the genetic control of longevity and age-related pathologies including protein homeostasis and folding. However, the available tools for studying the induction of autophagy and autophagic flux in worm are rather limited. In collaboration with the Miller Lab are working to develop sensitive, selective, cell lineage-specific probes for autophagy in the worm.
lipid probes
In collaboration with the Baker Lab, we are using protein engineering to design novel protein probes for the detection of specific lipids. Key technologies used in this project include protein active site redesign using Rosetta, assembly of bilayer nanodiscs, synthesis of new fluorescent lipids, and S. cerevisiae surface display.
Key papers
Nickerson DP and Merz AJ. 2015. LUCID: quantitative assay of ESCRT-dependent cargo packaging into the MVB. In press, Traffic.
Chapin HC, Merz AJ, Miller DL. 2015. Tissue-specific responses to aging and stress in C. elegans. Aging 7:419-434. —pdf
Nickerson DP, *Russell MRG, *Lo SY Milnes J and Merz AJ. 2012. Termination of isoform-specific Vps21/Rab5 signaling at endolysosomal organelles by Msb3/Gyp3. Traffic 13:1411-28. *Equal contributors. —pdf