Seattle, WA is the birthplace of long-term dialysis treatment. Prior to the 1960s, kidney failure was almost certainly a death sentence, but Drs. Belding Scribner, Wayne Quinton, and Lester Babb from the University of Washington (UW) pioneered chronic dialysis so that end-stage renal disease (ESRD) could became a manageable health condition. The first outpatient hemodialysis center was the Seattle Artificial Kidney Center (SAKC) founded in 1962 and located in the basement of Swedish Hospital.
While the breakthrough invention of dialysis enabled patients with ESRD to survive, dialysis technology has not significantly evolved since then. The Center for Dialysis Innovation (CDI) was formed in 2016 as another partnership between UW and SAKC (now Northwest Kidney Centers) to renew innovation in dialysis so that patients can go from surviving to thriving. The CDI is co-directed by Drs. Jonathan Himmelfarb (UW nephrologist) and Buddy Ratner (UW Bioengineering, Chemical Engineering) and is a collaborative effort across several UW departments and research teams. We are working on developing the Ambulatory Kidney to Improve Vitality (AKTIV), which we envision as a wearable, miniaturized dialysis system that is low-cost, offers simplistic blood access, and requires minimal anticoagulation.
Learn more about the Center for Dialysis Innovation.
Vascular Access
This work focuses on pro-healing biomaterial scaffolds for regenerating vascular tissue. Such scaffolds induce vascularization and reduce foreign body response, likely by modulating immune responses to the biomaterial. We are currently applying such scaffolds to a translational project on developing a novel vascular graft for blood access for patients on hemodialysis.
Keywords: precision porous, 6s, immunomodulation, pro-healing, vascular graft
Active Researcher(s): Le Zhen
Skin Interface
A critical component to enabling the portability of the AKTIV device is the development of a port system for vascular access. Since the port system will be directly connected to the vascular system, it must be infection-resistant and mechanically robust to avoid accidental removal. We are investigating the use of a percutaneous catheter cuff made of porous material to induce skin-healing at the blood access interface. Our material promotes skin regeneration which will restore the epithelial barrier to prevent infection around the access site. It also fully integrates with the skin to provide mechanical support.
Keywords: skin, regeneration, pro-healing, vascular access
Active Researcher(s): Meghan Wyatt
Blood Compatibility
Protein Adsorption
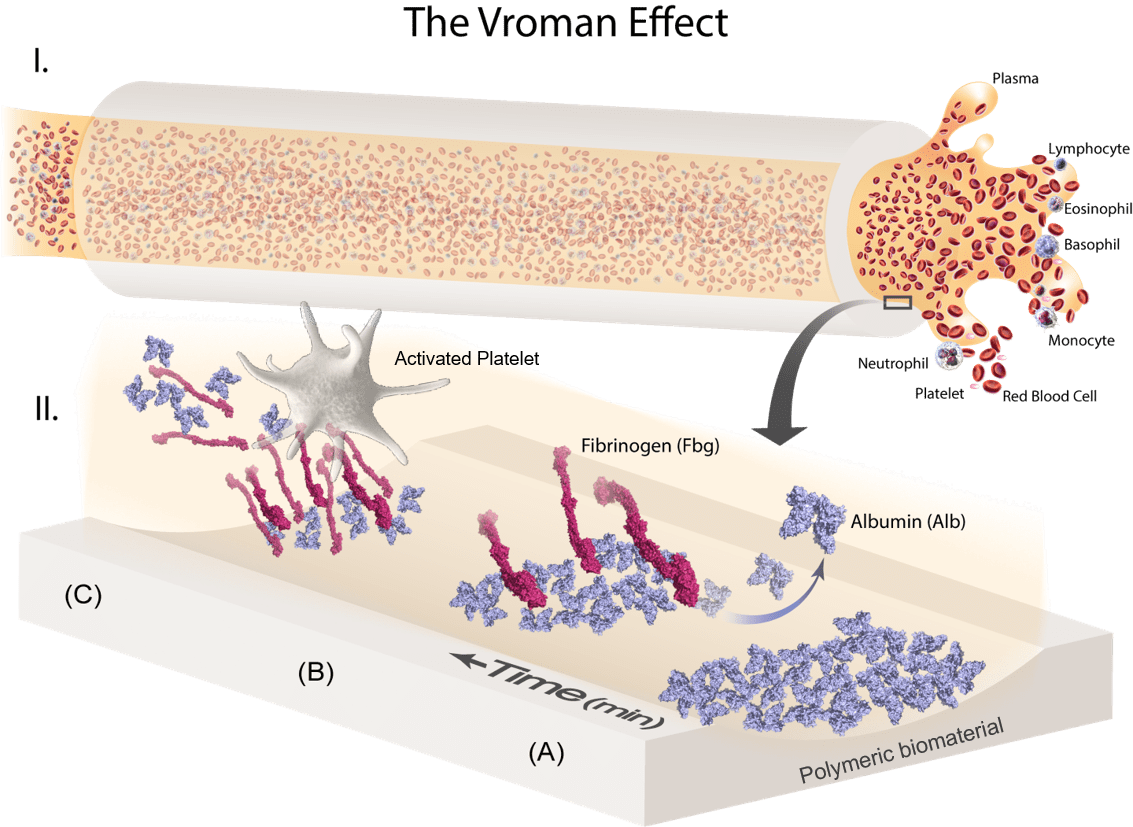
The surface adsorption and exchange of plasma proteins on blood-contacting biomaterials (Vroman Effect) is an initiating factor in determining the severity of the host pro-coagulation response. Thus, the goal of this project is to elucidate the adsorption/displacement mechanism and conformational changes of critical plasma proteins, specifically albumin (Alb) and fibrinogen (Fbg), in relation to various hydrophobic fluoropolymers (FPs) for the development of thromboresistant hemodialyzer surfaces. Adsorbed fibrinogen on blood-contacting surfaces has long been associated with platelet binding and aggregation, yet soluble fibrinogen remains unbound by circulating platelets in native bloodstream. This implies that fibrinogen in contact with artificial surfaces induces a conformational change that ‘switches on’ its bioactivity. Taken together, a molecular understanding of how a surface alters fibrinogen bioactivity is essential for the development of blood compatible coatings that can either (1) resist fibrinogen binding altogether, and/or (2) adsorb fibrinogen in a non-platelet binding conformation.
Magnified Illustration of a Blood-Contacting Catheter Lumen: Competitive plasma protein adsorption on foreign materials occurs upon exposure to blood and dictates subsequent pro-coagulation events (Vroman Effect). The immunogenically-inert protein, albumin (Alb), is the most abundant and mobile, and is kinetically driven to form a preliminary passivating layer against platelet adhesion. However, this layer is temporally displaced by fibrinogen (Fbg), a rod-like protein which denatures upon adsorption to expose integrin-binding epitopes that initiate platelet recruitment and fibrin clot formation. (Credit: Sherry Liu)
Low Blood Shear Regime
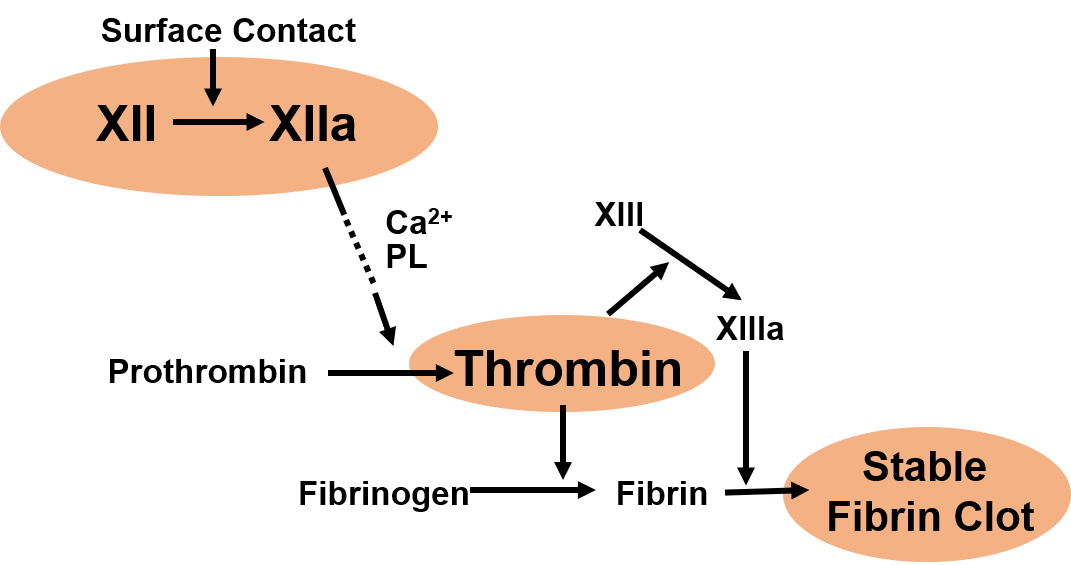
The ultimate goal of this project is to develop a comprehensive blood compatibility assay for surfaces exposed to stagnant to low shear hemodynamic flow. Consideration of shear rate brings up a different strategy to address blood compatibility, and the low-shear condition is predominantly governed by the intrinsic coagulation pathway, which is initiated by human factor XII adsorption and activation. Blood components involved in this pathway are being investigated to gain better understanding of molecular level events at the blood-surface interface. A wide variety of biomaterials have been studied to find the best surface property. The results from this study could contribute to the development of a blood-compatible surface upon exposure to low shear hemodynamic flow (e.g. hemodialyzer column head).
(Left) Simplified scheme of intrinsic coagulation pathway. (Right) Fibrin clot formed from human plasma upon contact with biomaterials. (Credit: Kyung-Hoon Kim)
High Blood Shear Regime
We are also investigating the effect of high blood shear rate on coagulation in medical devices. We hypothesize that a fluoropolymer surface can increase blood compatibility by creating a passivating layer of irreversibly bound platelets under high shear conditions. We have conducted in vitro experiments to confirm this hypothesis and plan to do animal studies to show that such a material would be a viable candidate for medical devices.
Keywords: fibrinogen, conformation, coagulation, fluoropolymer, blood shear, platelet binding, blood compatible, clot formation
Active Researcher(s): Sherry Liu, Kyung-Hoon Kim, David Lee