
Collaborations with Industry
We are currently collaborating with Genentech in the San Francisco Bay Area on projects related to chromatin regulation by methylation.
Synthetic Chemistry, Enzymology, and Cell Biology
Even the most complicated processes in our cells are based on the fundamental principles of physical-organic chemistry, and depend on the chemical reactivity of functional groups in biomolecules like Proteins, DNA and RNA. This makes organic chemistry a very powerful tool when it is carefully applied to understand how cells grow and repair themselves.
Our lab is particularly interested in understanding how simple chemical modifications of proteins, that are found from bacteria to humans, have dramatic effects on protein structure and function. We use a combination of organic synthesis and molecular biology to make semisynthetic proteins modified at specific amino acids. By studying the structure and function of these semisynthetic proteins under well-defined conditions that mimic living cells, we are learning how specific modified proteins work inside human cells.
Our diverse areas of research include:
1. Synthesis of small-molecule probes of enzyme function. (Denis Smirnov, B.S. ’14)
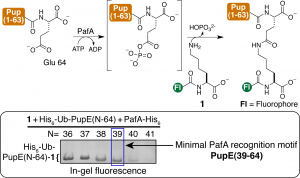
2. Development of new methodologies for chemically modifying intact proteins. (Caroline Weller, Ph.D. ’17)
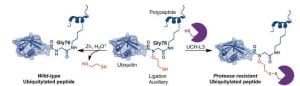
3. Synthesis of entire proteins with biologically active chemical modifications. (Abhinav Dhall, Ph.D. ’16)

4. Synthesis of protein-based probes of protease function. (Sam Whedon, Ph.D. ’18)

We are particularly interested in thiol-based chemistry for protein modification such as the oxidative elimination of cysteine, reversible disulfide formation, and the thiol-ene “click” reaction (shown below).

In order to chemically synthesize proteins with well-defined modifications at precise amino acid positions, we use a technique called Native Chemical Ligation (check out our Review article). This involves joining two halves of a protein by a peptide (amide) bond. One or both halves may be completely synthetic and can be made by solid-phase peptide synthesis. This allows us to incorporate any desired chemical modification in a protein including fluorescent dyes, isotopic labels for NMR studies, chemical and photoactivatable cross-linkers, and even radioactive elements for biological applications such as PET imaging.
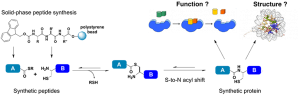
Basic techniques that students in our lab learn include, but are by no means limited to, small-molecule synthesis, solid-phase peptide synthesis, nuclear magnetic resonance (NMR) spectroscopy, native and denaturing gel electrophoresis, high performance and fast protein liquid chromatography. Depending upon the specific scientific problem a student is tackling, additional skills are acquired either within the lab or by collaborating with other research groups at UW.
Additional Reading Related to Our Research
From One Genome, Many Types of Cells. But How?
Generation of peptide thioesters for native chemical ligation
The Volcano Research Conference in Chemical Biology

Every year in early February our research group drives south from Seattle to Pack National Forest at the base of Mt. Rainier to attend the Volcano Conference in Chemical Biology. This two-day conference brings together leading Chemical Biologists from the Pacific Northwest in a rustic and awe-inspiring setting. Attended by ~120 folks from the U.S. and Canada, this conference highlights exciting new developments in the fields of bio-organic chemistry and chemical biology. The students and faculty enjoy listening to short talks by undergraduates/graduate students/post-docs as well as the plenary speaker. Afternoons are spent snow-shoeing in Rainier National Park and nights at the giant campfire listening to tall tales of experimental glory.
Past speakers from our labs: Rudy (’11), Denis (’12), AB, Meg & Caroline (’13), Caroline & Sam (’14), Sam (’15), Lena & Lars (’23).